Temperature effects
Undeniably there is an effect. A large one, but not something to be worried about. It has been said that all electronic devices are first and foremost temperature sensors. A sizeable proportion of the camera sensor noise generated within the Photonic Instrument is due to Johnson–Nyquist noise, which is highly temperature dependant. And some of our Photonic Instrument’s and the Null Gamma device’s entropy comes from that noise.
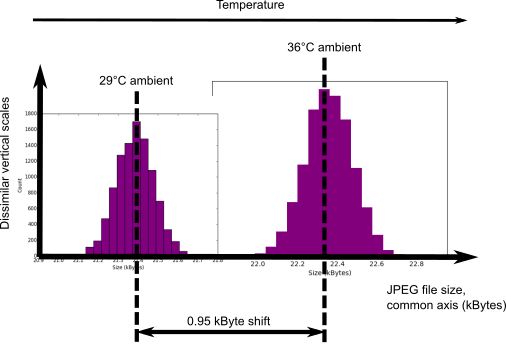
JPEG file size shift due to temperature change.
You can see from the above chart that a 7°C temperature rise causes the mean JPEG file size to shift upwards by $\approx 6 \sigma$ relative to the cooler measurements. And thus the entropy content of a single frame increases. This also has complicating implications for health checking of the instrument. The effect is surprisingly easy to mitigate though. You just have to know the signum of the temperature coefficient. We know that entropy generating rate changes relative to temperature changes are as $\frac{dH}{H} \sim \alpha dT$, where $\alpha$ is the temperature coefficient. But we don’t need to determine $\alpha$ at all. We just need to know whether it’s positive or negative which we can find from the literature or empirically. And then:-
If $\alpha > 0$, determine $H_{\infty}$ at the coolest likely temperature. Appropriate for camera sensors like inside the Photonic Instrument.
If $\alpha < 0$, determine $H_{\infty}$ at the warmest likely temperature. Appropriate for Zener diodes in avalanche mode loke inside the Zenerglass.
But you don’t conclusively know the signum of $\alpha$ for the complete entropy source circuit, so ideally you measure the entropy rate at minimum and maximum likely temperatures $(T_{min}, T_{max}) $ where $T_{min} < T_{max}$. A wrinkle; there is no guarantee at all though that the macro temperature coefficient of a complete entropy source is positive ($\alpha > 0$), negative ($\alpha < 0$) or even linear (when $ \alpha \Delta T \ll 1) $ .
Voilà. This technique (whilst time/calendar intensive) avoids any direct temperature control of the entropy source. As the ambient temperature changes, min. entropy has always been measured at the most entropy conservative point on the thermometer. Entropy can only increase from the baseline measurements. We’re excluding direct attacks on the instrument with liquid nitrogen as we’ll see the dudes in the room with the large steaming flask.